Psychedelic stocks were battered in premarket trading after a panel of experts advising the Food and Drug Administration rejected the approval of MDMA for post-traumatic (PTSD) stress disorder.
There were two votes - one for MDMA's efficacy and another for safety, by the FDA's Psychopharmacologic Drugs Advisory Committee. They voted 9-2 that MDMA – with talk therapy – is not effective for treating PTSD. The second vote was 10-1 that the benefits of MDMA treatment don't outweigh its risks.
This was the first time FDA advisers have considered a Schedule I psychedelic for medical treatment. Also, there has not been a new treatment for PTSD in over two decades, as more than 13 million Americans, many of who are veterans, suffer from the mental disorder.
The outcome of the two votes reflected panel members' struggle between new PTSD treatments and the clinical data submitted by drugmaker Lykos Pharmaceuticals. They pointed out the data had inconsistencies and unanswered questions and lacked evidence supporting the MDMA treatment used in the therapy sessions.
"It seems like there are so many problems with the data," Melissa Barone, one of the panelists and a psychologist with the VA Maryland Health Care System, told NPR News, adding, "Each one alone might be okay, but when you pile them up on top of each other..."
Several panel members raised concerns about allegations of potential misconduct and bias in the trials, which could have affected the results.
"I have real concerns with the validity of the data and the allegations of misconduct," said Elizabeth Joniak-Grant, a sociologist and a panel member, adding, "I can't in good conscience support something where these many harms are being reported."
However, Dr. Walter Dunn, a psychiatrist at UCLA, was one of the few panelists who voted in favor of MDMA.
Dunn acknowledged the misconduct allegations but added the data showed the treatment could be effective for PTSD.
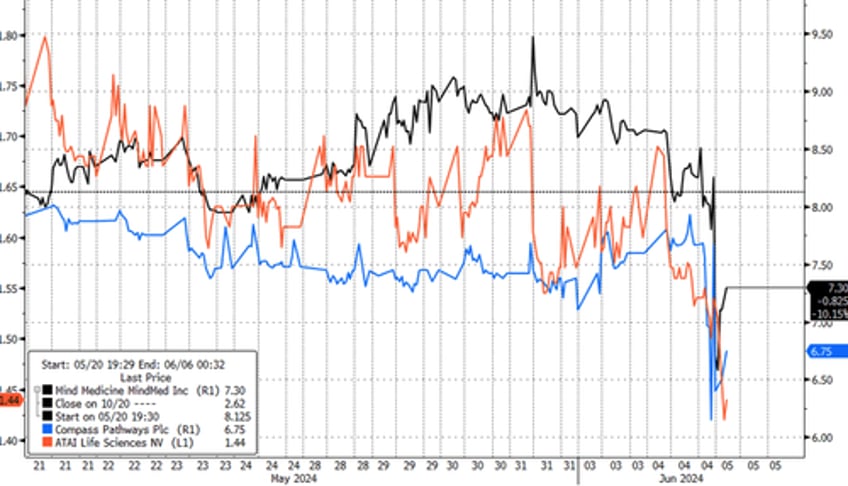
In premarket trading, psychedelic stocks were clubbed like a baby seal. Mind Medicine fell 14%, Compass Pathways down 11%, and Atai Life Sciences slid 8%.
Jefferies analyst Andrew Tsai wrote in a note, "Contrary to our expectation, the FDA AdCom voted negatively on Lykos' racemic MDMA having adequate data to demonstrate effectiveness in PTSD." He pointed out there are differences between Lykos' trial and other psychedelic programs, "we may see some increased uncertainty around the approvability of psychedelics."