Aspartame, the sweetener used as an ingredient in approximately 6,000 consumer foods and beverages sold worldwide has a dark history of controversy, political influence, and questionable science. Let’s dive into the scandal that shaped one of the most consumed food additives in the world.
Discovered accidentally in 1965 by James Schlatter at G.D. Searle, aspartame was touted as a miracle low-calorie sweetener. But its approval process revealed troubling findings, including animal studies showing harmful effects.
The first big red flag? In 1977, 98 of 196 infant mice exposed to aspartame died during an FDA investigation. The findings, later published as the Bressler Report, highlighted severe data manipulation and negligence by Searle. The report was kept under seal by the FDA for 3 decades.

Searle’s studies showed poor methodology to say the least.
Here are a few of the relevant findings summarized from various documents describing the FDA Task Force Report:
Excising masses (tumors) from live animals, in some cases without histologic examination of the masses, in others without reporting them to the FDA." (Schmidt 1976c, page 4 of US Senate 1976b) Searle's representatives, when caught and questioned about these actions, stated that "these masses were in the head and neck areas and prevented the animals from feeding.
"Failure to report to the FDA all internal tumors present in the experimental rats, e.g., polypsin the uterus, ovary neoplasms as well as other lesions."
Instead of performing autopsies on rhesus monkeys that suffered seizures after being fed aspartame, the company had financed a new monkey seizure study with a different methodology that showed no problems."
Reporting animals as unavailable for necropsy when, in fact, records indicate that the animals were available but Searle choose not to purchase them.
Animals which had died were sometimes recorded as being alive and vice versa. These include approximately 20 instances of animals reported as dead and then reported as having vital signs normal again at subsequent observation periods.
Selecting statistical procedures which used a total number of animals as the denominator when only a portion of the animals were examined, thus reducing the significance of adverse effects.
G.D. Searle told the FDA that 12 lots of DKP were manufactured and tested in one study, yet only seven batches were actually made. Significant deviations from the protocols of several studies were noted which may have compromised the value of these studies. In at least one study, the Aspartame 52 weeks monkey study, the protocol was written after the study had been initiated.
In each study investigated, poor practices, inaccuracies, and discrepancies were noted in the antemortem phases which could compromise the study.
This resulted in the FDA’s Chief Counsel suggesting a grand jury investigation.
“The Food and Drug Administration has recommended to the Justice Department that a grand jury be convened in Illinois to investigate charges that a major drug firm, G. D. Searle & Company, has falsified data and reports submitted in connection with new drug applications.”
So how did this substance that is obviously unsuitable for human consumption gain FDA approval?
Enter Donald Rumsfeld, former U.S. Secretary of Defense who was hired as the CEO by Searle in 1977. Rumsfeld promised to use his political influence to push aspartame through regulatory hurdles and boy did he keep his promise.
“When Searle was absorbed by Monsanto in 1985, Donald Rumsfeld reportedly received a $12 million bonus, pretty big money in those days. Also, while at Searle, Rumsfeld was awarded Outstanding CEO in the Pharmaceutical Industry from the Wall Street Transcript (1980) and Financial World”
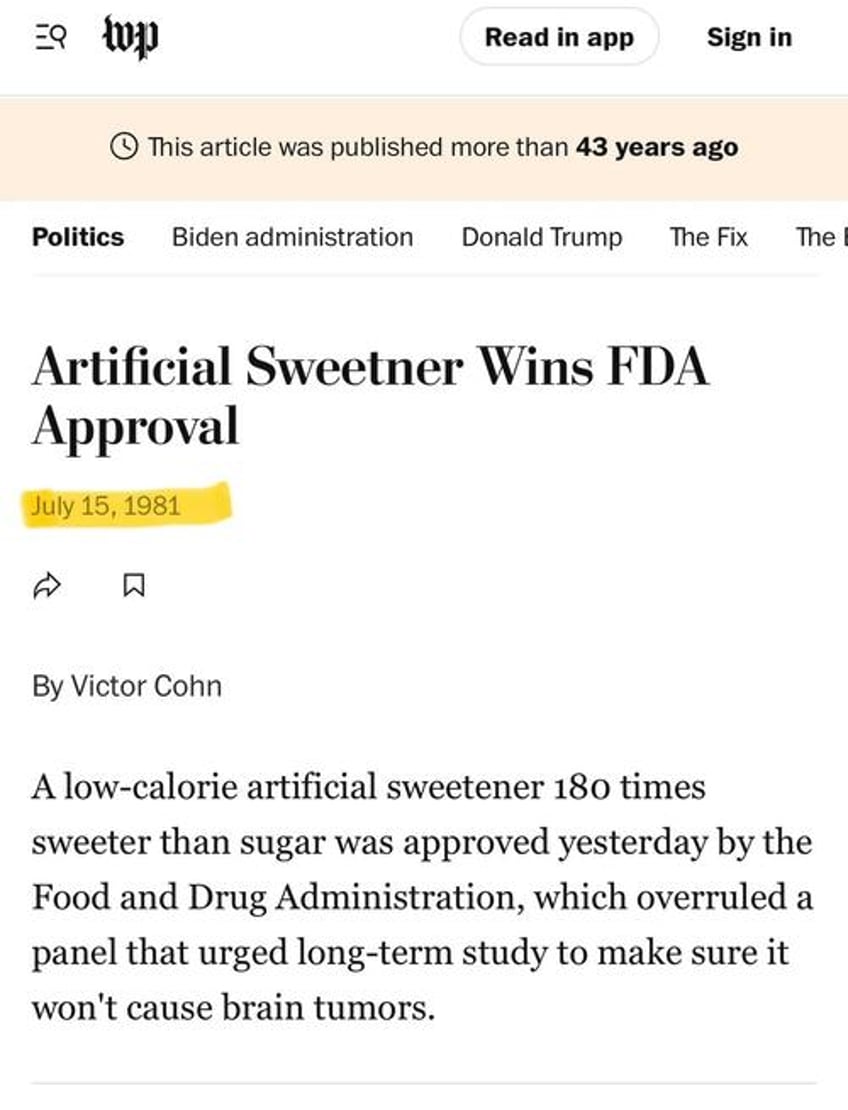
The pivotal moment came in 1981 when at the time Rumsfeld was part of Ronald Reagan’s transition team. After Reagan’s inauguration, he replaced the FDA Commissioner with Arthur Hull Hayes Jr., who approved aspartame within months.
From the article:
“The sweetener, aspartame, is safe, FDA Commissioner Arthur Hull Hayes Jr. ruled after reviewing all the evidence, including the scientific panel's recommendation for more study in animals.”
“Aspartame may be used, Dr. Hayes said, as a table-top sugar substitute, as a tablet or as an additive in cereals, drink mixes, instant coffee and tea, gelatins, puddings, fillings, dairy products and toppings.”
The 1981 Article announcing its approval:
This approval ignored the recommendations of an FDA Public Board of Inquiry (PBOI), which had concluded in 1980 that aspartame might cause brain tumors and should not be approved without further testing.
The FDA's own toxicologist, Dr. Adrian Gross who is the man who originally discovered the shortcomings of Searle’s studies told Congress that without a shadow of a doubt, aspartame can cause brain tumors or brain cancer and that it violated the Delaney Amendment, which forbids putting anything in food that is known to cause cancer. Here is what Gross told a 1987 Senate Committee Hearing:
“… no amount of additional examinations of pathology material such as undertaken by the UAREP … [or] … new additional statistical analyses … and no judgmental evaluations or interpretations of any data arising from those studies can in any way rectify the basic problem …: in the absence of reasonable expectation that the experimental animals were administered the correct dosages of the test agent, any observational data carried out on those animals must be regarded as questionable or flawed. This is to say nothing of all the myriad of other problems involving the competence of those conducting such studies, and the [lack of] care they exercised in their execution. Once a study is carried out and the test animals are disposed of, all that remains are the number of tiny bits of tissue preserved from their organs for microscopic examination and the written records of observations made by those who actually carried out that study. While the tissues themselves can be examined by others long after the remains of those animals no longer exist, the reliability of the written records has already been found to be unacceptable in a great variety of ways. … Once a study is compromised in its executions, it is beyond salvation by anyone. Even with respect to those small portions of tissue preserved for microscopic examination for an indefinite period of time after any study is completed there are serious problems … there is little if any assurance that such samples of tissues as were preserved actually originate from the specific animals said … to have been their source … Furthermore, due to the unacceptably high rate of post-mortem autolysis, a great many such tissues were not collected at all from the experimental animals.”
Key studies, E33 and E70, entitled SC-18862: Two Year Toxicity Study in the Rat: Final Report showed alarming results. Rats fed aspartame had higher rates of brain tumors compared to controls. Yet, these were brushed aside during the approval process.
Hayes even admitted, “I’m not prepared to say there is no risk from aspartame,” but greenlit it anyway for dry foods in 1981. By 1983, he approved it for carbonated beverages too, opening the door to massive consumption.
Searle’s lobbying power didn’t stop there. Investigators found that multiple FDA officials involved in the approval later joined companies tied to aspartame production or lobbying—raising serious concerns about conflicts of interest.
Arthur Hull Hayes Jr. himself after resigning from the FDA in 1983, joined Burson-Marsteller, the public relations firm representing G.D. Searle, the company that developed aspartame.
Michael Friedman: Serving as FDA Deputy Commissioner, Friedman defended aspartame’s safety during the 1990s. In 1999, he left the FDA to become a senior vice president at G.D. Searle.
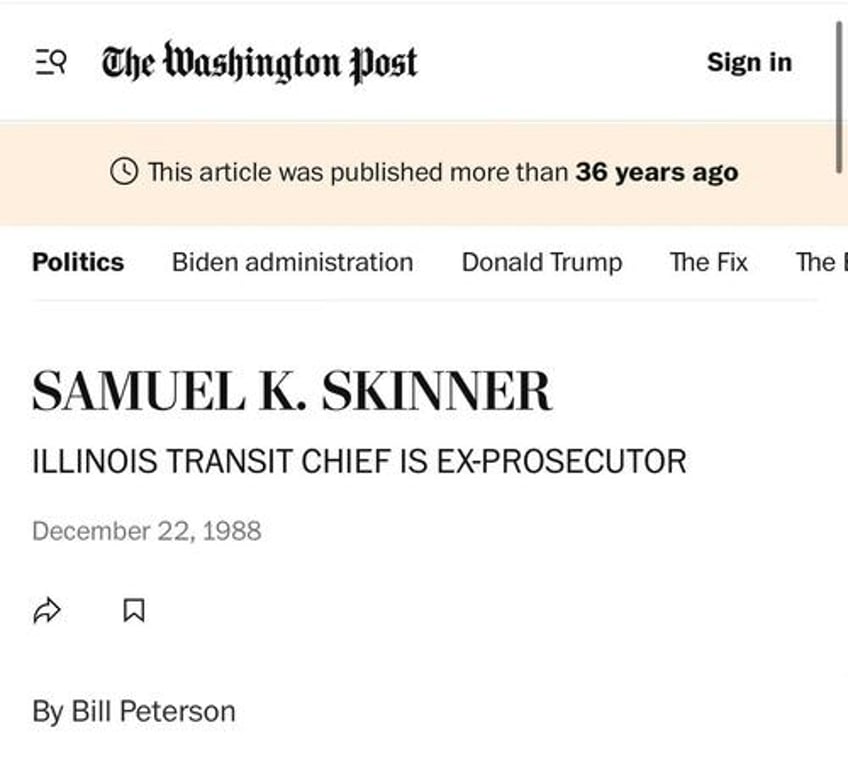
Samuel Skinner whose nickname was “Sam the Hammer”, while a U.S. Attorney Skinner was assigned to investigate G.D. Searle for alleged data falsification related to aspartame studies. During the investigation, he entered employment negotiations with Sidley & Austin, Searle’s law firm, and subsequently withdrew from the case before joining the firm.
Sherwin Gardner: As FDA Deputy Commissioner, Gardner signed the initial approval for aspartame in 1974. He resigned from the FDA in 1979 to become Vice President of the Grocery Manufacturers of America, an industry group with members involved in aspartame’s use.
Aspartame’s breakdown in the body was another issue. It metabolizes into methanol, formaldehyde, and phenylalanine. Critics like Dr. Woodrow Monte warned about potential neurotoxicity and methanol poisoning.
“The consumption of aspartame sweetened soft drinks or other beverages in not limited by either the consumption of aspartame sweetened soft drinks or other beverages in not limited by either calories or Osmolality, and can equal the daily water loss of an individual (which for active people in a calories or Osmolality, and can equal the daily water loss of an individual (which for active people in a state like Arizona can exceed 5 liters). The resultant daily methanol intake might then rise to unprecedented levels. Methanol is a cumulative toxin and for some clinical manifestations it may be an unprecedented levels. Methanol is a cumulative toxins and for some clinical manifestations it may be a human-specific toxin.”
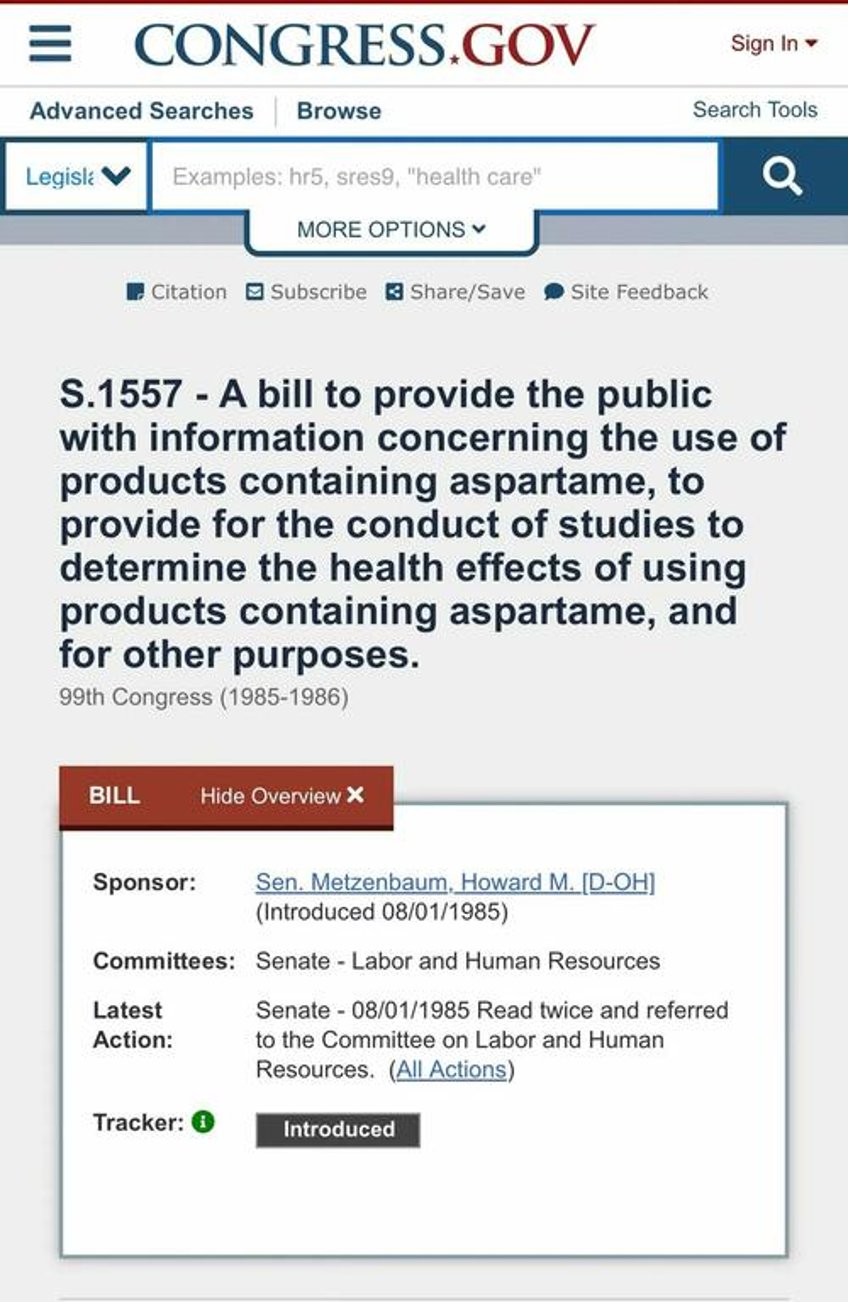
In soft drinks, aspartame’s instability leads to higher methanol release, especially when exposed to heat. Yet, these risks were downplayed by both regulators and manufacturers. Meanwhile, public opposition grew. Senator Howard Metzenbaum proposed the Aspartame Safety Act in 1985, which aimed to mandate clearer labeling and further studies. It failed due to industry lobbying.
Critics like neuroscientist Dr. John Olney also pointed to a spike in brain cancer rates since aspartame’s approval, correlating it with its widespread use in soft drinks. However all of these warnings were not heeded and aspartame continue to be consumed daily by millions of Americans.
Aspartame consumption exploded in the U.S., peaking in the 1980s and 1990s. By 1987, 17M pounds were consumed annually, mostly in diet sodas. By 1983, aspartame had become a $336M industry for Searle. NutraSweet, its brand name, dominated the market, with Coca-Cola and Pepsi signing contracts to use it in diet sodas.
In 2021, the global aspartame market was valued at $375.5 million. It is projected to reach $1.86 billion by 2030.
Despite public health concerns, NutraSweet’s marketing positioned aspartame as a safe, modern alternative to sugar. The FDA insisted it was safe for most consumers, dismissing calls for updated testing.
Watch this creepy commercial that aired back then.
Today, critics argue that aspartame’s safety evaluations rely too heavily on industry-funded studies. Independent research, like Dr. Soffritti’s Ramazzini study, has linked it to cancers in animals.
The moral of the story? Don’t consume this stuff, it can cause cancer and poison you and your family.
Finally here is a brief news story that aired on Fox5 years ago exposing Aspartame’s troubling background.
Ask yourself, if this was allowed with just one additive, how many others are there with a similar story?
Hidden in plain sight, quietly contributing to the epidemic of autoimmune disease, cancers and general health problems the nation as a whole is suffering.
Sources:
1. The Bressler Report
Full Report:
https://revolutionhealth.org/blog/wp-content/uploads/2025/01/Bressler-Report-on-Aspartame.pdf
2. NYT article:
3. Rumsfeld Article
https://www.huffpost.com/entry/donald-rumsfeld-and-the-s_b_805581/amp
4. EFSA’s toxicological assessment of aspartame: was it even-handedly trying to identify possible unreliable positives and unreliable negatives?
Full Study:
https://pmc.ncbi.nlm.nih.gov/articles/PMC6628497/
5. Aspartame Article
https://vtechworks.lib.vt.edu/server/api/core/bitstreams/a814bc16-5c18-…
6. 1995 The Cancer Letter
https://cdn.cancerhistoryproject.com/media/1999/06/10000000/TCL25-24.pdf
7. Samuel Skinner Article
8. Aspartame: Methanol & the Public Health
Full Study: https://shorturl.at/mSAMF
9. Aspartame Bill
https://www.congress.gov/bill/99th-congress/senate-bill/1557
10. Increasing Brain Tumor Rates: Is There a Link to Aspartame?
Full Study:
https://academic.oup.com/jnen/article-abstract/55/11/1115/2610500?redir…
11. Aspartame Market Research
https://www.alliedmarketresearch.com/aspartame-market-A11795
12. First experimental demonstration of the multipotential carcinogenic effects of aspartame administered in the feed to Sprague-Dawley rats
Full Study: