
The Supreme Court is set to hear oral arguments on Tuesday, March 26, in a case that could have a significant impact on how mifepristone — the first drug used in a two-drug medication abortion regimen — is used and prescribed in the United States.
The case, FDA v. Alliance for Hippocratic Medicine, surrounds the U.S. Food and Drug Administration’s (FDA) rolling back of safety restrictions for mifepristone, including its 2016 action extending the permissible gestational age of the baby for which a girl or woman may take abortion drugs from seven weeks gestation to ten weeks gestation and its 2021 rule change allowing abortionists to send mifepristone through the mail. The FDA also recently made permanent its rule to allow women and girls to receive a prescription for mifepristone via telemedicine.
The Alliance Defending Freedom (ADF) filed the lawsuit in November of 2022 against the FDA on behalf of four national medical associations and several doctors, alleging the agency “chose politics over science and approved chemical abortion drugs for use in the United States.”
“The U.S. Food and Drug Administration (FDA) — the federal agency responsible for ensuring the safety of drugs that Americans take — has betrayed women and girls,” Erik Baptist, senior counsel with the ADF and co-counsel on the case, said during a press conference in Washington, DC, on Thursday.
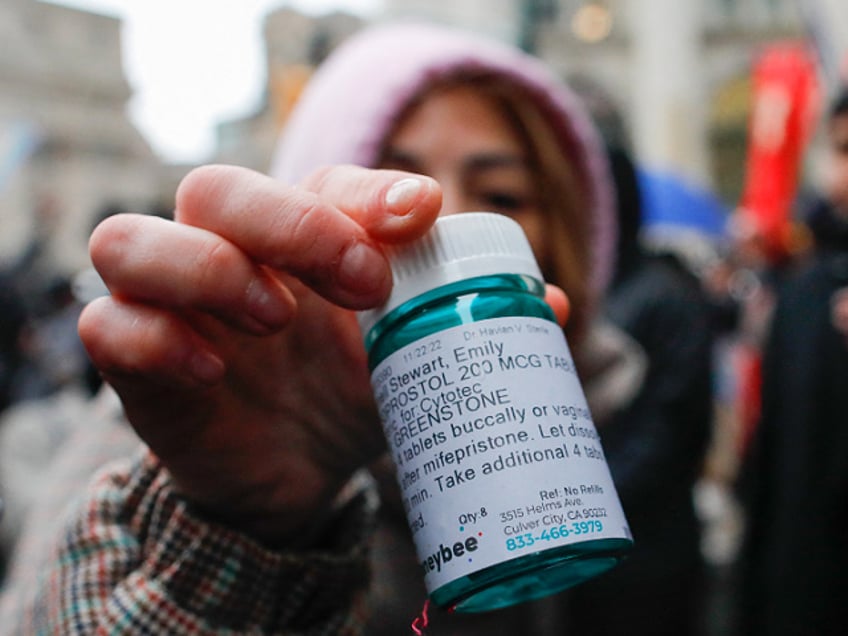
A pro-abortion activist displays abortion pills as she counterprotests during an anti-abortion demonstration on March 25, 2023, in New York City. (KENA BETANCUR/AFP via Getty Images)
“Women should have the ongoing care of a doctor when taking high risk drugs. But in recent years, the FDA has recklessly removed nearly every safeguard the agency previously considered necessary for abortion drugs, including in-person doctor visits to check for ectopic pregnancies, severe bleeding, and life-threatening infections,” Baptist added. “Without question, the FDA’s actions made taking high-risk abortion drugs less safe for women.”
Justices agreed to take up the case in December of 2023. In September, both President Joe Biden’s Department of Justice (DOJ) and Danco Laboratories LLC, which distributes mifepristone under the brand name Mifeprex, asked the Supreme Court to reverse a lower court decision halting two FDA actions that loosened restrictions on the abortion pill. The Supreme Court ultimately granted a hearing to both cases, consolidating them into one with a total of one hour allotted for oral argument.
Biden’s DOJ and Danco Laboratories appealed an August ruling from the U.S. Court of Fifth Circuit, which found that the FDA’s 2016 decision to allow the abortion pill to be taken up to ten weeks of pregnancy instead of seven weeks is unlawful. The court said the same of the FDA’s 2021 rule change, which allowed the abortion pill to be mailed directly to patients and allowed medical professionals other than doctors to prescribe mifepristone.
“In loosening mifepristone’s safety restrictions, FDA failed to address several important concerns about whether the drug would be safe for the women who use it,” reads the panel opinion, penned by Judge Jennifer Walker Elrod.
“It failed to consider the cumulative effect of removing several important safeguards at the same time. It failed to consider whether those ‘major’ and ‘interrelated’ changes might alter the risk profile, such that the agency should continue to mandate reporting of non-fatal adverse events,” the opinion continued. “And it failed to gather evidence that affirmatively showed that mifepristone could be used safely without being prescribed and dispensed in person.”
Elrod wrote that at the preliminary stage, plaintiffs “have made a substantial showing” that the 2016 and 2021 rule changes violate the Administrative Procedure Act (APA).
Despite the Fifth Circuit’s ruling against the government and the drug manufacturer, mifepristone has remained available under existing regulations while the litigation continues. The Supreme Court preemptively paused any ruling from an appeals court this spring, pending a petition for the Supreme Court to take the case.
The ADF’s lawsuit points to six discrete agency actions since the legalization of mifepristone and misoprostol in 2000. The ADF alleges that the agency was only able to approve the drug by falsely classifying pregnancy as an “illness.” The lawsuit also alleges that the FDA never studied the safety of mifepristone under the labeled conditions of use, ignored the potential impacts of the hormone-blocking regimen on the developing bodies of adolescent girls, disregarded evidence that chemical abortion drugs cause more complications than surgical abortion, and eliminated necessary safeguards for pregnant girls and women who take the regimen.
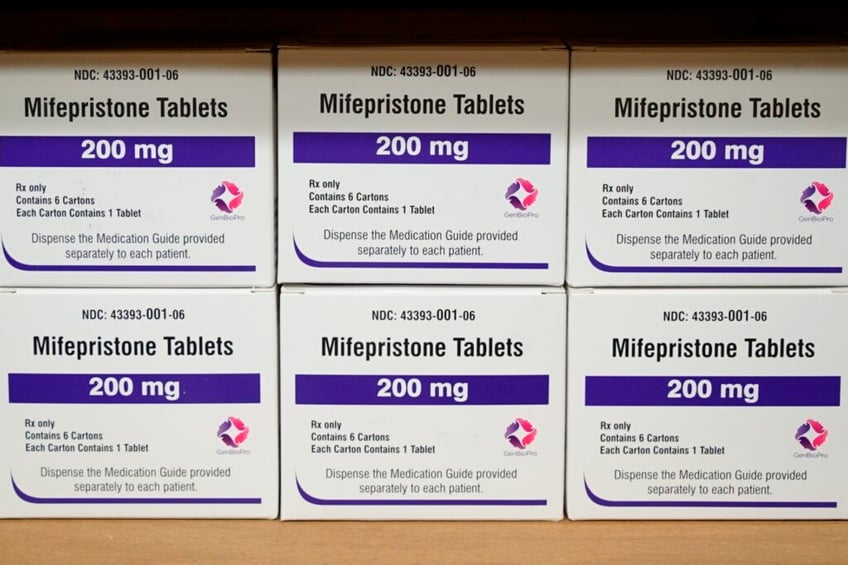
Boxes of the drug mifepristone sit on a shelf at the West Alabama Women’s Center in Tuscaloosa, AL, on March 16, 2022. (AP Photo/Allen G. Breed, File)
The lawsuit details how, in 2016, the FDA extended the permissible gestational age for which a girl or woman may take the abortion drugs. Then, in 2021, the FDA allowed abortionists to send mifepristone through the mail, which the ADF says was “in direct violation of federal law.”
The complaint alleges:
All of the FDA’s actions on chemical abortion drugs — the 2000 approval, the 2016 major changes, the 2019 generic drug approval, and the two 2021 actions to eliminate the in-person dispensing requirement — failed to acknowledge and address the federal laws that prohibit the distribution of chemical abortion drugs by postal mail, express company, or common carrier. Instead, the FDA’s actions permitted and sometimes even encouraged these illegal activities.
The Fifth Circuit took up the case after U.S. District Judge Matthew Kacsmaryk of the U.S. District Court for the Northern District of Texas handed down a 67-page decision that the FDA’s decisions were illegal under federal law, issuing a nationwide injunction blocking the abortion pill.
Days later, the U.S. Court of Appeals for the Fifth Circuit partially granted a stay requested by the Biden Justice Department. The court’s 42-page opinion temporarily put on hold the part of the decision about the 2000 FDA decision because it might be past the deadline for bringing legal challenges, though added that it was a “close call” and that the court might go the opposite direction after receiving additional legal arguments. But the appellate court rejected a stay on anything from 2016 to the present, affirming the trial court’s injunction.
The Supreme Court then put a hold on Kacsmaryk’s entire decision. As Breitbart News previously noted, an administrative stay is not in any way a reflection on the legal merits of the case. A Supreme Court justice can issue one just to preserve the status quo while the court is receiving arguments from both sides.

Three members of the Women’s March group protest in support of access to abortion medication outside the Federal Courthouse on Wednesday, March 15, 2023 in Amarillo, Texas. (AP Photo/David Erickson)
The Supreme Court declined to take up an appeal from the ADF asking justices to rule on the legality of the FDA’s 2000 approval of mifepristone.
In a medication abortion, mifepristone blocks the action of progesterone, which the mother’s body produces to nourish the pregnancy. When progesterone is blocked, the lining of the mother’s uterus deteriorates, and blood and nourishment are cut off to the developing baby, who then dies inside the mother’s womb. The drug misoprostol (also called Cytotec) then causes contractions and bleeding to expel the baby from the mother’s uterus.
This week. the pro-abortion Guttmacher Institute released a report which found that medication abortions accounted for 63 percent of all abortions within the formal U.S. health care system in 2023 after the Supreme Court overturned Roe v. Wade — meaning an estimated 642,700 unborn babies died in medication abortions. The percentage is up from an estimated 53 percent in 2020 and 39 percent in 2017. The report did not account for abortion pills obtained through underground national and international networks, including those that send pills to women in red states.
The Biden administration has relentlessly promoted medication abortions, and in January of 2023, the FDA allowed retail pharmacies to dispense abortion pills. At the same time, several Democrat governors have stockpiled abortion pills while enacting shield laws that protect health care providers who prescribe mifepristone to women and girls in states with laws protecting the unborn.
The case is FDA v. Alliance for Hippocratic Medicine, No. 23-235 in the Supreme Court of the United States.
Katherine Hamilton is a political reporter for Breitbart News. You can follow her on X @thekat_hamilton.
