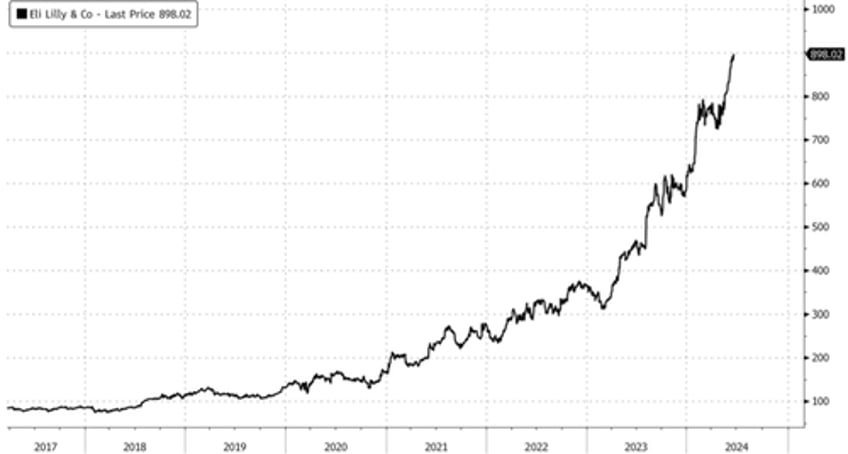
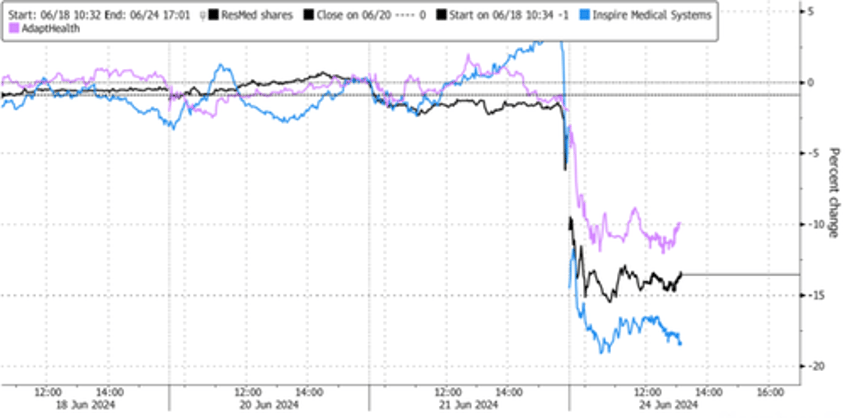
Shares of medical equipment companies specializing in sleep apnea treatments nosedived on Monday after a new study from Eli Lilly and Company revealed that its tirzepatide weight-loss drug significantly reduced the severity of sleep apnea.
On Friday, Eli Lilly announced new results from its SURMOUNT-OSA phase 3 clinical trials evaluating tirzepatide injection to treat moderate-to-severe obstructive sleep apnea, otherwise known as OSA.
Tirzepatide, branded as Mounjaro (a diabetes drug) and Zepbound (a weight loss drug), reduced moderate-to-severe OSA in obese adults with and without positive airway pressure (PAP) therapy.
Eli Lilly and Company (NYSE: LLY) announced detailed results from the SURMOUNT-OSA phase 3 clinical trials evaluating tirzepatide injection (10 mg or 15 mg) for the treatment of moderate-to-severe obstructive sleep apnea (OSA) in adults with obesity, with and without positive airway pressure (PAP) therapy.
In both studies, tirzepatide achieved all primary and key secondary endpoints for both the efficacy and treatment-regimenii estimands and demonstrated a mean reduction of up to 62.8% on the apnea-hypopnea index (AHI), or about 30 fewer events restricting or blocking a person's airflow per hour of sleep, compared to placebo.
"In the trials, patients with moderate-to-severe obstructive sleep apnea and obesity treated with tirzepatide experienced about 30 fewer disruptive events every hour of sleep and nearly half achieved disease resolution," said Atul Malhotra, MD, Peter C. Farrell presidential chair, professor of medicine at University of California San Diego School of Medicine and director of sleep medicine at UC San Diego Health.
Malhotra continued, "OSA can be very disruptive to daily life and affects a person's long-term health when left untreated because it can lead to serious cardiometabolic complications. These data support the efficacy of tirzepatide in adults living with moderate-to-severe OSA and obesity and has the potential to add to our toolbox for OSA treatment."
Louis Aronne, an obesity specialist at Weill Cornell Medicine, told Bloomberg that Eli Lilly's phase 3 clinical data of tirzepatide is a "breakthrough" for the "treatment of obesity and sleep apnea."
In markets, Eli Lilly's shares rose 2% by the early afternoon trade in the US. BofA analysts noted earlier that the magnitude of OSA resolution from tirzepatide treatment "was not baked into investors' expectations," with the firm maintaining a 'Buy' rating and $1,000 price target.
Meanwhile, Citi analysts downgraded ResMed, which makes breathing machines known as CPAP devices, to 'Neutral' from 'Buy' on the tirzepatide development. Shares on Monday of ResMed plunged 15%, along with Inspire Medical Systems -17% and AdaptHealth -11.5%
Citi analysts also said the data indicates that GLP-1 is a viable treatment option for the 70% of OSA patients who are obese.