Eli Lilly has launched a wave of cease-and-desist orders targeting compounding pharmacies, telehealth companies, and medical spas that produce and sell "compounded" versions of tirzepatide, the active ingredient in the pharma giant's diabetes drug Mounjaro and weight-loss wonder drug Zepbound.
As early as December 2022, Tirzepatide was placed on the Food and Drug Administration's shortage list. The shortage designation allowed pharmacists, doctors, and licensed outsourcing facilities to compound copycats of Zepbound amid the GLP-1 craze that swept across America. It was removed from the list last Wednesday.
Bloomberg noted Lilly began targeting telehealth companies and medical spas with cease-and-desist letters on Thursday.
Here's an excerpt from the letter, first obtained by Bloomberg: "Because Lilly's FDA-approved medicines are available, you must immediately cease any production, sale, dispensing and marketing" of compounded copies.
The latest estimate from Fierce Pharma shows as many as two million Americans have been taking "copycat versions of Eli Lilly and Novo Nordisk's incretin hormone drugs, compounding pharmacies have suddenly thrived from their ability to help meet the booming demand for these products."
After the FDA removed Lilly's tirzepatide, the Outsourcing Facilities Association, a compounding industry group, sued the federal agency.
More from Fierce Pharma...
In the US District Court in Fort Worth, Texas, the Outsourcing Facilities Association (OFA) has alleged (PDF) that the FDA's action was contrary to law and that it should be immediately revoked. The OFA also is seeking a temporary order that would prevent the FDA from taking action against its members for making compounded versions of tirzepatide until the lawsuit is resolved.
The suit criticizes the agency for not soliciting public comment on the move and for taking the word of Lilly that it can meet the demand for the products. The suit said that Lilly "is self-interested in monopolizing the market."
"The agency's decision will have tremendous implications across the nation for patients and physicians, as well as the outsourcing facilities that made an enormous investment to meet patient demand in light of product shortages and delays," Lee Rosebush, the chairman of the OFA, said in a release.
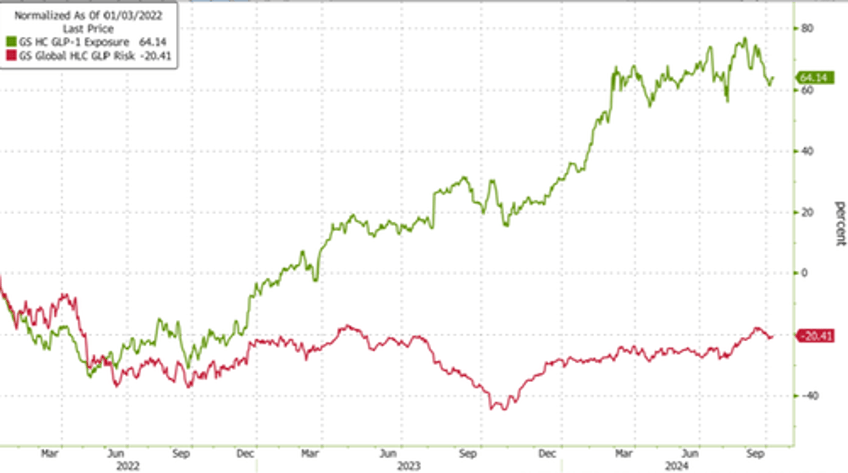
Tracking the GLP-1 craze in markets, Goldman's index of companies with high exposure to GLP-1s shows momentum in these stocks has widely stalled throughout 2024 as companies at risk from GLP-1s' success have been clawing back losses.
There is mounting evidence that GLP-1s like tirzepatide are wonder drugs for obesity and diabetes. Wall Street's looming question is whether the pharmaceutical company can keep up with demand following the FDA's action last week.